Answer: atomic
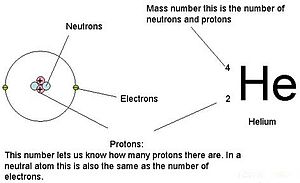
The number of protons plus neutrons in an atom is its number. The atomic number or proton number (symbol Z) of a chemical element is the number of protons found in the nucleus of every atom of that element. Ninjatrader for mac download. The atomic number uniquely identifies a chemical element. It is identical to the charge number of the nucleus. Atomic number is the number of protons in the nucleus of an atom. It can also be the number of electrons in a neutral atom. Pay attention to the word neutral. The proton number is so important that it is used to identify an element.

Most relevant text from all around the web:
Number Of Protons In An Atom Is Equal To
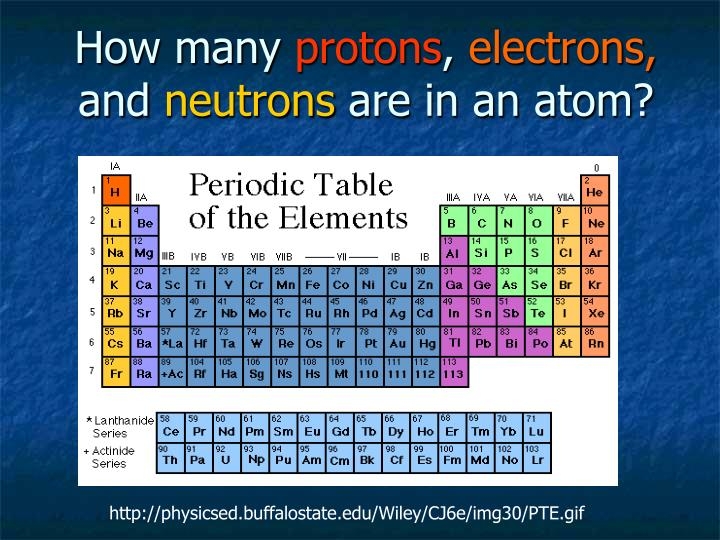 The number of protons in at atom is its ________ number. The atomic number or proton number (symbol Z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The atomic number uniquely identifies a chemical element. It is identical to the charge number of the nucleus. In an uncharged atom the atomic number is also equal to the number of electrons. The sum of the atomic number Z and the number of neutrons N gives the mass numberA of an atom. Sin… The number of protons in an atom is called its atomic number . Ernest Rutherford (1919) observed that nitrogen under alpha-particle bombardment ejects what appeared to be hydrogen nuclei. By 1920 he had accepted that the hydrogen nucleus is a distinct particle within the atom … Atom - Wikipedia Mass number - Wikipedia Atomic number - Wikipedia Atomic number - Wikipedia In chemistry the number of protons in the nucleus of an atom is known as the atomic number which determines the chemical element to which the atom belongs. For example the atomic number of chlorine is 17; this means that each chlorine atom has 17 protons and that all atoms with 17 protons are chlorine atoms. The chemical properties of each atom are determined by the number of (negatively charged) electrons which for neutral atoms is equal to the number of (positive) protons so that the total charge i… All atoms above atomic number 82 (82 protons lead) are radioactive. There are three main types of radioactive decay; alpha beta and gamma. Alpha decay is when the atom shoots out a particle having two protons and two neutrons. This is essentially a helium nucleus. mass number (symbol: A) which is the sum of the number of protons and number of neutrons in the nucleus of an atom relative atomic mass (also called atomic weight ; symbol: A r ) which is the ratio of the average mass per atom of ..
The number of protons in at atom is its ________ number. The atomic number or proton number (symbol Z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The atomic number uniquely identifies a chemical element. It is identical to the charge number of the nucleus. In an uncharged atom the atomic number is also equal to the number of electrons. The sum of the atomic number Z and the number of neutrons N gives the mass numberA of an atom. Sin… The number of protons in an atom is called its atomic number . Ernest Rutherford (1919) observed that nitrogen under alpha-particle bombardment ejects what appeared to be hydrogen nuclei. By 1920 he had accepted that the hydrogen nucleus is a distinct particle within the atom … Atom - Wikipedia Mass number - Wikipedia Atomic number - Wikipedia Atomic number - Wikipedia In chemistry the number of protons in the nucleus of an atom is known as the atomic number which determines the chemical element to which the atom belongs. For example the atomic number of chlorine is 17; this means that each chlorine atom has 17 protons and that all atoms with 17 protons are chlorine atoms. The chemical properties of each atom are determined by the number of (negatively charged) electrons which for neutral atoms is equal to the number of (positive) protons so that the total charge i… All atoms above atomic number 82 (82 protons lead) are radioactive. There are three main types of radioactive decay; alpha beta and gamma. Alpha decay is when the atom shoots out a particle having two protons and two neutrons. This is essentially a helium nucleus. mass number (symbol: A) which is the sum of the number of protons and number of neutrons in the nucleus of an atom relative atomic mass (also called atomic weight ; symbol: A r ) which is the ratio of the average mass per atom of ..
Number Of Protons In An Atom Determines Its
Disclaimer:
Our tool is still learning and trying its best to find the correct answer to your question. Now its your turn, 'The more we share The more we have'. Comment any other details to improve the description, we will update answer while you visit us next time..Kindly check our comments section, Sometimes our tool may wrong but not our users.
Are We Wrong To Think We're Right? Then Give Right Answer Below As Comment
