What Is A Mole?
This video covers what moles are, and how to create conversion factors for them. It also explains why your teacher is doing this 'moles thing' to you, which is a question I've heard from many a frustrated chem student. Trust me, there is a reason!
Converting Grams To Moles
Lots of great grams-to-moles and moles-to-grams action in this one. Moles are confusing at first, but once you see it's always a one-step problem, it gets nice and repetitive (and that's a good thing!). Also intriguing: what to do about molecules with crazy parentheses?
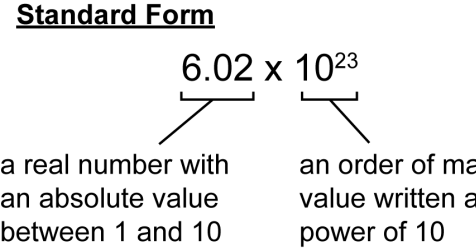
Avogadro's Number (6.02 x 1023)
These mole problems get the most confusing when Avogadro's Number enters the mix: lots of scientific notation, calculators going crazy. This video will make Avogadro make sense for you, though, and it covers the common calculator mistake that's responsible for almost all of the errors students make in this type of problem.

Converting Grams to Number of Atoms or Molecules
The hardest problems are the ones going from grams all the way to number of atoms or molecules. The big trick that helps the most students involves looking at it as a basic stepping stone problem with 'moles in the middle', as I'll show you in this video. Also covered: a few more advanced moles problems with funky wording to give you a taste of what's to come.
Help With Scientific Notation
Dvd burner download for mac. Avogadro's number Avogadro's number is the number of particles in one mole of any substance. Its numerical value is 6.02225 × 1023. One mole of oxygen gas contains 6.02 × 1023molecules of oxygen, while one mole of sodium chloride contains 6.02 × 1023sodium ions and 6.02 × 1023 chloride ions. Use Avogadro's number to find the mass of a single atom. Group coefficients together and exponents together to divide numbers in scientific notation. Avogadro’s number is defined as the number of elementary particles (molecules, atoms, compounds, etc.) per mole of a substance. Office 2011 mac download. It is equal to 6.022×10 23 mol-1 and is expressed as the symbol N A. Avogadro’s number is a similar concept to that of a dozen or a gross. A dozen molecules is 12 molecules. A gross of molecules is 144 molecules. Wattpad for mac free download. The Avogadro's number is equal to (6,022 x 10 raised to 23 particles) and is symbolized in the formulas with the letters L or NA. In addition, it is used to make conversions between grams and atomic mass unit. The unit of measure of the Avogadro's number is the mole (mol-1) but it can also be defined in lb/mol-1 and oz/mol-1. Avogadro's number is defined as the number of atoms present in one mole of any substance. Avogadro's number is equal to 602,200,000,000,000,000,000,000. Since it is a large number therefore Avogadro used scientific notation to express the value for easy understanding. Therefore, scientific notation of Avogadro's number is.
